Ingredient Details
Vonos has been making headlines because of its versatility, effectiveness, and approval by the EPA for use against SARS-CoV-2, the novel coronavirus that causes COVID-19. But is Vonos safe? What are the ingredients? And what makes Vonos different from other products?
Use in HVAC Systems
Vonos is designed to be used as apart of a comprehensive HVAC and duct
maintenance program. The purpose of such a program is to assure that the HVAC system and
ducts function in the manner they were designed to, remain free from odor-causing mold,
microbial growth, and other contamination.
FAQs
Commonly asked questions and answers about Vonos.
What are EPA product numbers and EPA establishment numbers?
How to read EPA product numbers and EPA establishment numbers.
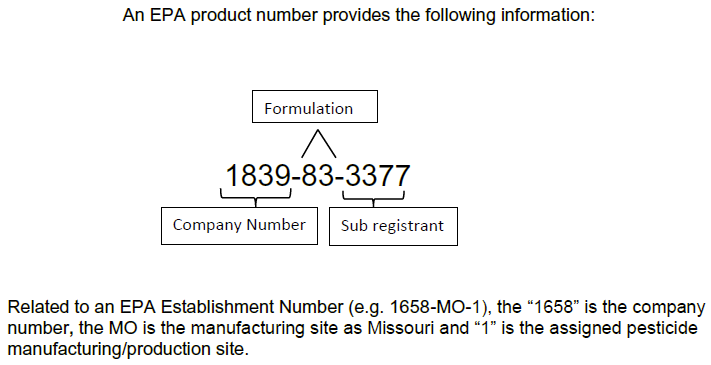
Vonos EPA number 82972-1-96360
What is the dilution ratio for vonos?
Please follow directions carefully before use.
FULL STRENGTH
VONOS comes ready to use (RTU) as a full-strength EPA hospital disinfectant for non-porous surfaces. Full strength is recommended for first-time use on surfaces. Full strength should also be used in environments with high rates of bacteria, mold, and mildew and in high traffic areas and touch points.
5:1 CARPET ANO RUG SANITIZER
To Sanitize and Deodorize Carpets and Rugs:
• Mix 5-parts water to 1-part VONOS.
• Spray onto surface from a distance of 6-8 inches.
• Work the product down to carpet pad (brush may be used)
• Allow enough ventilation to air dry.
• Repeat process as needed.
First test for color fastness in an inconspicuous space. Do not use on wool fabrics. If applying after use of an oxidizer for cleaning and spotting, extract and rinse oxidizer first.
9:1 FOOD-CONTACT & DAILY SURFACE SANITIZER
To Sanitize Food-Contact Surfaces:
• Mix 9-parts water to 1-part VONOS.
• Spray onto surface from a distance of 6-8 inches, wetting thoroughly.
• Let stand for 1 minute (or longer if specified by government sanitary code)
• Allow surface to drain or air dry. Do not rinse or wipe.
To Sanitize Non-Food-Contact, Non-Porous Surfaces:
• Mix 9-parts water to 1-part VONOS.
• Spray onto surface from a distance of 6-8 inches, wetting thoroughly.
• Let stand 5 minutes.
• Wipe and allow to air dry. No rinse required.
Is chlorine dioxide some sort of bleach?
No. While chlorine dioxide has chlorine in its name, its chemistry is very different from the corrosive chemistry of chlorine bleach. The primary differences are that chlorine dioxide is less caustic, safer, and gentler than bleach and many other antiseptics and antimicrobials, plus it remains effective under organic load. Further, chlorine bleach produces harmful by-products to the environment, including trihalomethanes (THM) and haloacetic acids (HAAS). VONOS breaks down to a simple salt, producing no harmful by-products.
What does it mean that a disinfectant is labeled green?
The United States Environmental Protection Agency (EPA) is currently reviewing the way third parties will be able to carry “green” claims on all disinfectant labels. EPA policy at this point does not allow “green” claims to be placed directly on any disinfectant product’s label. While VONOS is mild on skin, hard surfaces, and fabric, and will certainly qualify for “green status” when the designation is allowed, we cannot advertise this claim until it is permitted by the EPA. VONOS is also a powerful disinfectant able to kill some of the toughest and most resistant forms of bacteria and mold. The chemical composition of VONOS is such that it has a minimal impact on the environment and contains no ozone harming volatile compounds (VOC).
What is a Hospital Grade Disinfectant?
As part of the EPA registration process, disinfectant products are put through rigorous testing to prove their efficacy and measure toxicity. The EPA registers three types of disinfectants: Limited, General, and Hospital. All three disinfectants destroy or irreversibly inactivate certain microorganisms on hard, inanimate surfaces and objects. You can determine a “limited,” “general,” or “hospital” disinfectant by the microorganisms listed on the label.
Limited must be supported by efficacy testing against either Salmonella cholerasuis or Staphylococcus aureus. Limited disinfectants are found mostly in household use.
General must be supported by efficacy testing against both Salmonella cholerasuis and Staphylococcus aureus. General disinfectants are used in commercial areas.
Hospital must be supported by AOAC Use Dilution or AOAC Germicidal Spray efficacy testing against Staphylococcus aureus, Salmonella cholerasuis and Pseudomonas aeruginosa. The bacteria Pseudomonas aeruginosa hides behind biofilm and is difficult to eliminate. Killing this bacterium is required for “Hospital Disinfectant”.
Also, as part of this evaluation process, products are assigned to a toxicity category: The categories range from category 1 (highly toxic) to category 4 (no exposure warnings required on the label). VONOS received an EPA category 4 rating for all exposure routes with the exception of mild eye irritation.
What is the difference between a food-contact sanitizer and a non-food- contact sanitizer?
A food-contact sanitizer, at a minimum, reduces the level of Staphylococcus aureus and Escherichia coli by 99.999% on a food contact surface within one minute. A potable water rinse is not allowed after sanitation of a food-contact surface.
A non-food-contact sanitizer, at a minimum, reduces the level of Staphylococcus aureus and Klebsiella pneumoniae or Enterobacter aerogenes by 99.9% on non-food contact surfaces within 5 minutes.
What does "no rinse required on food contact surface" mean?
“No rinse required on food contact surfaces” is a safety rating given by NSF International (previously the National Sanitation Foundation). The NSF testing guidelines are a continuation of the USDA product approval and listing program, including the FDA 21. VONOS is rated “no rise required on food contact surfaces” category D2, meaning VONOS is approved for use in commercial or residential kitchens to control bacteria, viruses, and mold without the need to wash/rinse the area with water after VONOS is applied.
Storage Details
Transportation of Vonos should be done with only plastic pumps with the transferred material being stored in an opaque containers to guard against UV light and labeled with secondary container labels. Both packages bulk and transferred must be stored according to the label instructions. Please do not mix drum pumps with any other chemicals. This may cause a potential chemical reaction between the different chemistries.
STORAGE AND DISPOSAL – Store in original container in a cool, dry, place away from heat and open flame. Do not allow product to become overheated in storage. Avoid prolonged storage temperatures above 90F. This may cause increased degradation of the product, which will decrease product effectiveness. If you have any questions please feel free to reach out to us via email at [email protected] or via phone at 888-698-6667.
